Johnson & Johnson (JNJ) Vaccine Candidate Enter Into 3rd Phase, With Analysts Forecast

Johnson & Johnson (NYSE:JNJ) has moved into phase 3 development of its coronavirus vaccine candidate. The study, called Ensemble, will enroll up to 60,000 volunteers across three continents.
The one-shot format will also mean that Johnson & Johnson will be able to get initial efficacy data more quickly measured from the start of enrollment to readout because it won't have to wait until trial participants get booster shots before starting to count cases of COVID-19 among them.
Johnson & Johnson says it hopes to have enough data from the study to allow it to gain FDA emergency use authorization for the vaccine in early 2021. Investors should keep in mind that the healthcare conglomerate has committed to selling the vaccine on a not-for-profit basis, so regardless of how it might ultimately fare in the COVID-19 vaccine market, it won't affect the company's earnings.
Analysts Forecast
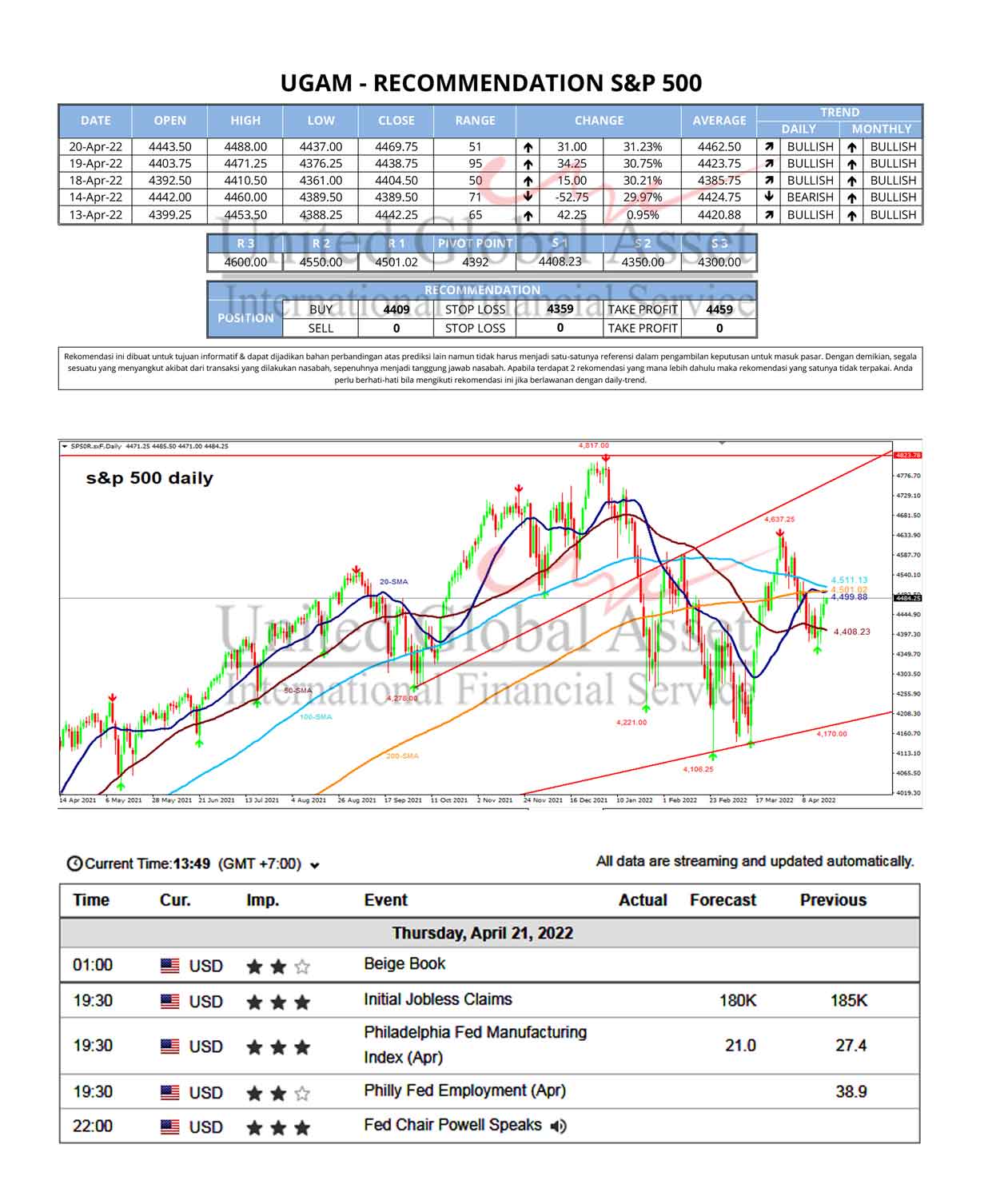
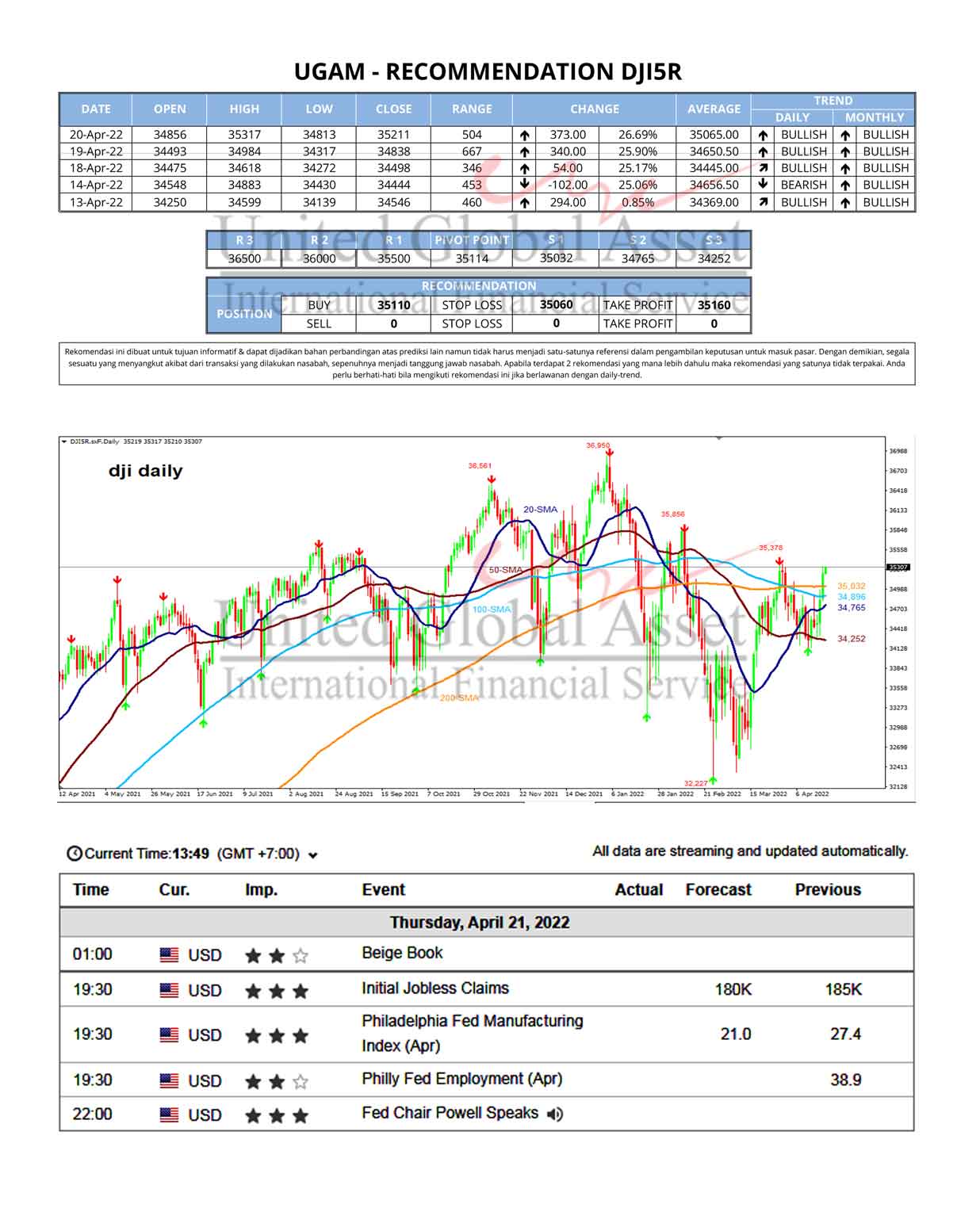
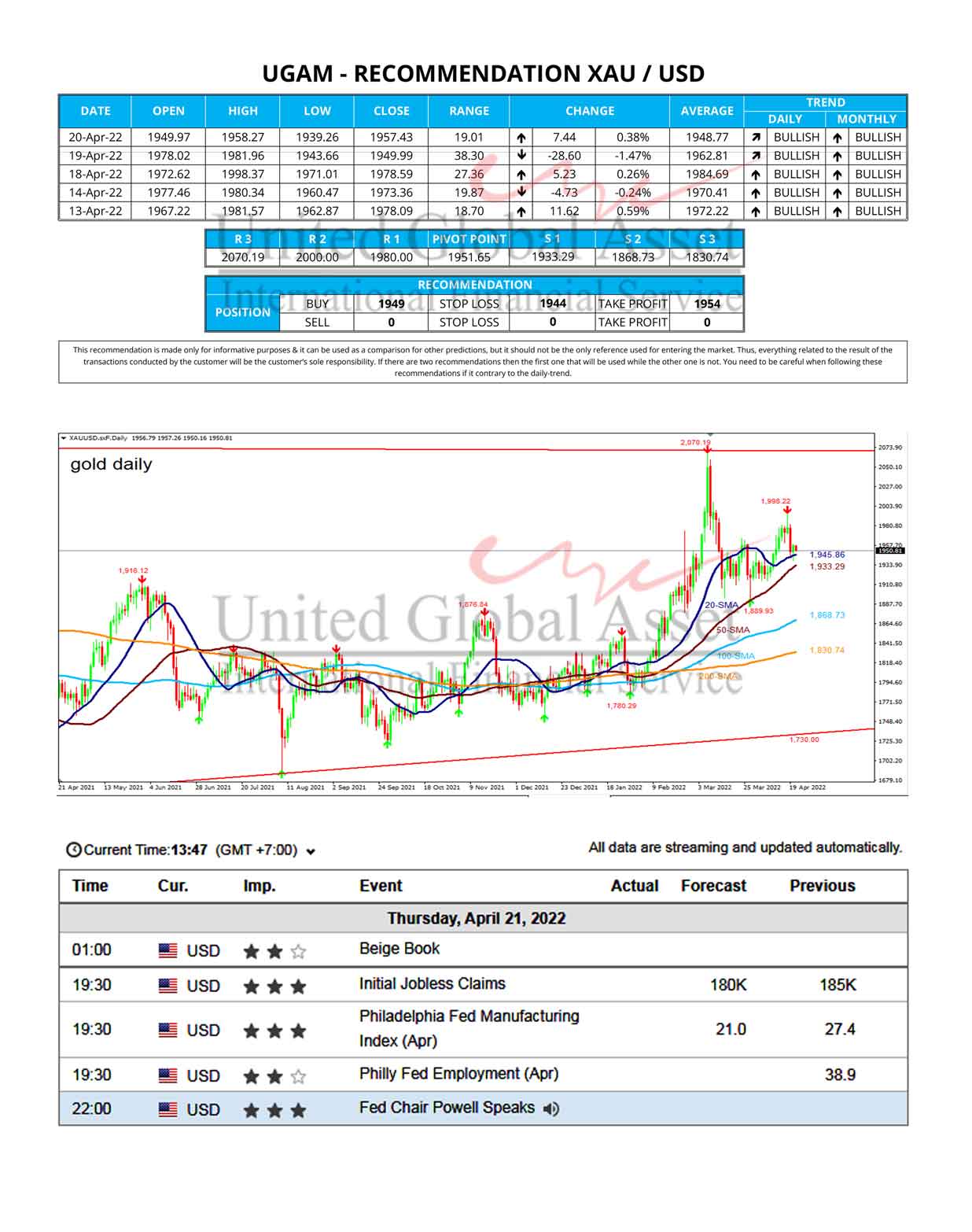
The 15 analysts offering 12-month price forecasts for Johnson & Johnson have a median target of 170.00, with a high estimate of 180.00 and a low estimate of 142.00. The median estimate represents a +17.74% increase from the last price of 144.38.
Analysts Recommendation
The current consensus among 17 polled investment analysts is to buy stock in Johnson & Johnson. This rating has held steady since August, when it was unchanged from a buy rating.